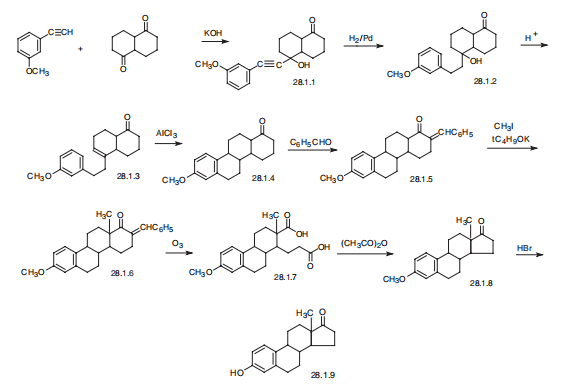
一些古早方法
A convenient synthesis of progesterone from stigmasterol
https://pubs.acs.org/doi/abs/10.1021/jo00442a044
A Synthesis of Progesterone from Ergosterol
https://pubs.acs.org/doi/10.1021/ja01610a036
The Preparation of Dehydroandrosterone from Cholesterol
https://pubs.acs.org/doi/10.1021/ja01311a036
The Synthesis of Estradiol and 1-Methylestradiol from Cholesterol
https://pubs.acs.org/doi/10.1021/ja01215a001
Butenandt, A., & Hanisch, G. (1935). Über Testosteron. Umwandlung des Dehydro-androsterons in Androstendiol und Testosteron; ein Weg zur Darstellung des Testosterons aus Cholesterin. Hoppe-Seyler´s Zeitschrift Für Physiologische Chemie, 237(1-3), 89–97. doi:10.1515/bchm2.1935.237.1-3.89
butenandt1935.pdf (1012.7 KB)
Ruzicka, L., & Wettstein, A. (1935). Sexualhormone VII. Über die künstliche Herstellung des Testikelhormons Testosteron (Androsten-3-on-17-ol). Helvetica Chimica Acta, 18(1), 1264–1275. doi:10.1002/hlca.193501801176
Synthesis of testosterone from dehydroandrosterone as described by Butenandt and Hanisch in 1935
https://eje.bioscientifica.com/view/journals/eje/180/6/EJE-19-0071.xml
Sondheimer, F., Mechoulam, R., & Sprecher, M. (1964). The synthesis of 10-hydroxy-10α-testosterone. Tetrahedron, 20(11), 2473–2485. doi:10.1016/s0040-4020(01)90827-0
Watanabe, Y., Mizuhara, Y., & Shiota, M. (1971). Synthesis of 19-hydroxy-19a-methyl-5-ene steroids via the 6.beta.,19-epoxy derivatives. The Journal of Organic Chemistry, 36(17), 2558–2560. doi:10.1021/jo00816a043
Kovganko, N. V., Kashkan, Z. N., & Chernov, Y. G. (1992). Synthesis of 19-hydroxysteroids I. New synthesis of 19-hydroxytestosterone. Chemistry of Natural Compounds, 28(6), 584–588. doi:10.1007/bf00630434
EHRENSTEIN, M., BARBER, G. W., & GORDON, M. W. (1951). INVESTIGATIONS ON STEROIDS. XIII. TRANSFORMATION OF STROPHANTHIDIN INTO COMPOUNDS STRUCTURALLY RELATED TO STEROID HORMONES1. The Journal of Organic Chemistry, 16(3), 349–375. doi:10.1021/jo01143a002
Mgr. Vojtěch Kapras
Syntéza a vlastnosti neuroaktivních steroidů
Synthesis and Properties of Neuroactive Steroids
PhD. Thesis